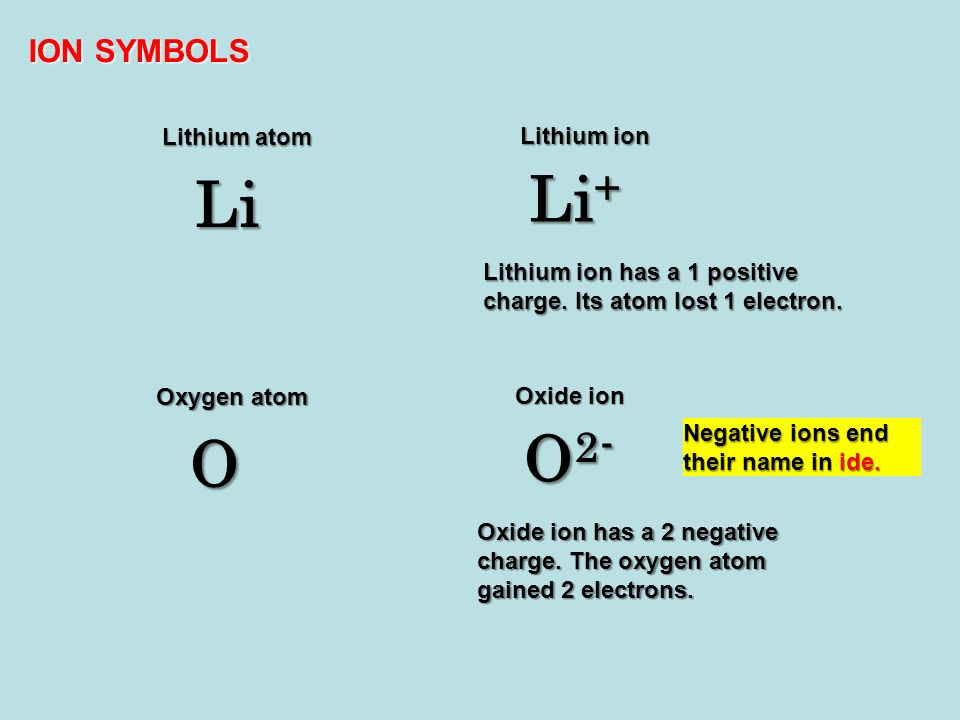
When an Atom of Lithium Loses an Electron
Option C The atom of lithium becomes positively charged ion. It can lose one of its electrons making it an ion.

Emerging Redox Flow Battery Technology For Grid Scale Storage Flow Battery Energy Storage Nanotechnology
When an atom loses an electron it becomes an ion that is positively charged and in this process the atom will lose its mass also as there is some mass of electron too.

. Loss of an electron is called. When metals loses the electrons positive ions are formed. Gaining an electron results in a negative charge so the atom is an anion.
What happens if sodium loses a valence electron. On StudySoup on 5312017. When helium atom loses one electron it becomes positive ion with 1 charge.
If a lithium atom loses an electron it becomes a lithium cation Li. Losing an electron results in a positive charge so atom ion is a cation. Thus there are three electrons in a lithium atom.
Lithium has 3 electrons 2 in inner shell and 1 in outer shell. What is lithium called when it loses an electron. 3 question At about 302 an atom of Lithium loses an electron to an atom of Fluorine.
Positive ion with a radius smaller than the radius atom loses an electron means less Identify the element in the table classified as a. If it loses an electron it becomes a positive ion see page 10 for more on ions. When an atom gainsloses an electron the atom becomes charged and is called an ion.
When lithium loses an electron from its outermost orbit it forms the positive ion called lithium ion Li. The nonmetals accept these electrons and form negative ions. When a helium atom loses electrons it get positive charge because the number of protons in the helium atom becomes greater than the number of electrons.
Consequently what charge do lithium ions have. These positive and negative ions attract each other through electrostatic force and form the bond called ionic bond. When a neutrally charged atom loses an electron to another atom the result is the creation of.
When an atom of lithium loses an electron the atom becomes a 1 negative ion with a radius smaller than the radius of the atom 2 negative ion with a radius larger than the radius of the atom 3 positive ion with a radius smaller than the radius of th. And when an atom loses an electron it becomes a positive ion because electrons are negative charged. Why does a lithium atom.
Losing an electron results in a positive charge so atom ion is a cation. Why are there and - signs on the elements after the electron is transferred. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6-1.
This 20 words question was answered by John B. Also electrons are negatively charged so in losing an electron the lithium ion would become positive. Therefore it is a positive ion.
1 mole of lithium li is equal to how many atoms of lithium. This is because now the. Moreover when it loses two electrons its become positive ion with 2 charge on it.
When an atom of lithium loses an electron the atom becomes. Gaining an electron results in a negative charge so the atom is an anion. When an atom gainsloses an electron the atom becomes charged and is called an ion.
Since lithium can only lose one electron and will have an excess proton its charge would be 1. The atom then loses or gains a negative charge. These atoms are then called ions.
Lig Lig e. Elements on the left side of the periodic table tend to lose electrons and those on the right side gain electrons. A potassium atom loses one electron to form a ion.
If lithium loses one electron then it is said to have a charge of 1 if 2 electrons lost then the charge would be 2 and if all the electrons are lost then charge would be 3. Of course the alkali metals and lithium is one are good reducing agents because this reaction is facile. Because mass and charge are always conserved in any chemical reaction if lithium or indeed ANY METAL loses an electron a Li will result ie.
When an atom loses an electron what happens. Positive Ion Occurs when an atom loses an electron negative charge it has more protons than electrons. How many electrons are in a neutral atom of.
When an atom of lithium loses an electron the atom becomes a When an atom loses an electron it becomes a positive ionWhen an atom of lithium loses an electron and becomes an ion it has one less electron shell so its radius is smaller than the radius of the atom. Mass of an electron 91 10-31 Kg So if an atom lose one electron then 9110-31. It would be A because Lithium only has 3 electrons in total by losing one electron it would lose the only electron in its outer shell of electrons and therefore become smaller in size.
Do you see this. Why or why not. A because a lithium ion originally has two shells with the one electron.
Is this a chemical reaction. When an atom loses an electron the atom becomes a positively charged ion. Because Lithium atom has has 3 electrons and if it loses an electron itll lose the electron in its 2s shell which means that atom will become smaller in size.
It now has more positive protons than electrons so it has an overall positive charge. When an atom of lithium loses an electron the atom becomes a positive ion with a radius smaller than the radius of the atom. When the metal loses an electron it changes from a metal to an ion.
Remember that when an atom loses electron it forms an ion with a positive charge called cation. A lithium atom has no electrical charge while a lithium cation has a charge of 1 because it loses an electron. To see more answers head over to College Study Guides.
A lithium atom has 3 protons and 3 electrons.

Ionisation Energy And The Periodic Table Siyavula Textbooks Grade 10 Physical Science Openstax Cnx

Ions Atoms Are Neutral But When An Atom Gains Or Loses An Electron It Becomes An Ion Ions Can Be Positive Or Negative Ppt Download

Starter Activity 04 05 2019 1 Atom 2 Element 3 Compound 4 Mixture Ppt Download
Rothenburg St Jakob Hochaltar Li Flugel Verkundigung Heimsuchung 1466 Annunciation Detail Of Fa Renaissance Furniture Ancient Tools Drill Press Table

Lithium Atom An Overview Sciencedirect Topics

Valency Of Lithium How Many Valence Electrons Does Lithium Li Have

5 7 Periodic Properties Of The Elements Chemistry Libretexts Element Chemistry Ionic Radius Electron Configuration

Ionic Bonding Infographic Diagram With Example Of Ionic Bond Between Lithium And Fluorine Atoms Showing Ionization Energy And Electron Affinity For Chemistry Science Education Royalty Free Cliparts Vectors And Stock Illustration Image

The Year In Chemistry 2017 Science Infographics Chemistry Classroom Learning

Lithium Art Print By Carlos Clarivan Atomic Structure Electron Configuration Atom

Tom Lehrer Interesting Fellow Teaching Chemistry Chemistry Lessons Nursing Student Tips

Nobelium Art Print By Carlos Clarivan Electron Configuration Atom Atomic Structure

Ions Ionic Bonding O Level Chemistry Notes

A Polyatmoic Ion Chart Allows A Chemist To See The Combinations And Formula S For Compounds Chemistry Lessons Science Chemistry Study Chemistry
The Periodic Table Ions Ppt Download
Organic Chemistry Foundational Concepts Of Organic Chemistry Atomic Structure Nucleus And Electrons Wikibooks Open Books For An Open World

Ionic Compounds Igcse Chemistry Quizizz

Comments
Post a Comment